Soba lab
Research Overview
The development and maintenance of neuronal networks is the basis for brain function. To develop and properly connect our intricate network of the estimated 86 billion neurons in the human brain requires precise regulation of wiring in time and space. Yet how neurons develop their individual morphology, find the right partners and maintain functional connectivity allowing us to learn and remember throughout life is still not well understood.
To get insight into these questions we use Drosophila melanogaster as a model system. The approximately 12.000 functional neurons in the larval brain and over 130.000 in the adult fly, form a simple yet sufficiently complex nervous system to produce a range of behaviors supporting growth and survival, which we can study with high precision. The high degree of conserved molecular functions further allows us to deduce general principles of network formation and function and to extrapolate the results to higher organisms. In particular, we study the peripheral nervous system (PNS) and its circuitry. The PNS consists of regularly arranged sensory neurons lining the larval body wall. Each sensory neuron class features stereotyped morphology and specific functions, ranging from mechanosensation to nociception.
We currently focus on using the nociceptive network in this system, which detects noxious stimuli (e.g. heat and touch) resulting in a stereotyped escape response. Sensory dendrites of nociceptive neurons cover the entire larval body-wall and are also an excellent system to address basic mechanisms of circuit development and function. The downstream network is surprisingly complex, and we use it to study circuit development, maintenance and sensory integration at the molecular, cellular, functional and behavioral level.
Due to the high degree of conservation of disease-relevant genes between flies and humans (~70 %) this system offers a highly relevant and efficient model to study genetic mechanisms underlying neurodevelopmental disorders as well as early neurodegenerative processes.
Maintenance of circuit integrity and synaptic specificity
Juvenile organisms like Drosophila larvae maintain the functional connectivity of their network through all stages, while increasing their body surface 100-fold within few days. This requires immense growth of dendrites and circuit-specific synapse addition to maintain receptive field coverage and functional output, respectively.
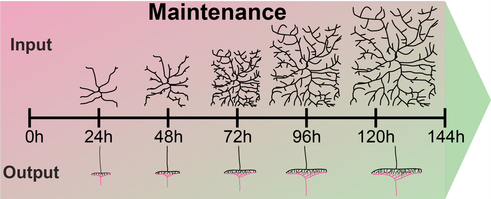
Figure 1: Dendrite and synapse growth scales with larval development to maintain receptive field coverage and functional output, respectively.
How is this coordinated growth regulated at the organismal and cellular level? We have identified two distinct classes of regulators, which are required to sustain neuronal morphology and synaptic connectivity of sensory neurons.
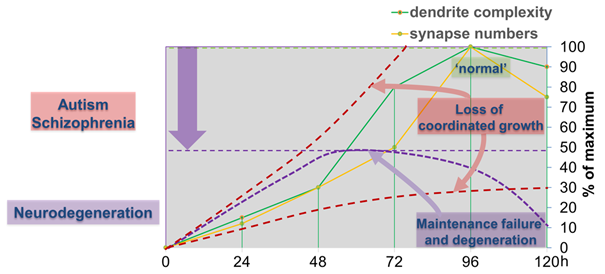
Figure 2: Dendritic and synaptic growth during larval development is highly regulated. Loss of coordinated growth due to aberrant cellular signals skews morphology and connectivity, which are hallmarks of neurodevelopmental disorders like autism and schizophrenia. Alternatively, failure to maintain developmental dendrite and synapse growth due to loss of gene function can result in growth arrest and eventually degeneration of synapses, axons and dendrites.
The first class is required for coordinated growth to keep neuronal and synaptic growth rates in sync with larval development (Tenedini et al. 2019, Hu et al. 2020, 2022, Chen et al. 2023). Genes regulating this process also seem to be required for circuit specific addition of synapses. We are investigating the underlying signaling machinery to gain mechanistic insight into the regulation of growth synchronization and synaptic specificity. This work is of particular relevance as abnormal connectivity has been linked to neurodevelopmental disorders including autism and schizophrenia.
We have also identified a second class of regulators, which are required to sustain neuronal morphology and synaptic connectivity of sensory neurons during larval development. Some of these regulators cause axon length-dependent synapse loss due to inflammatory cytokine-induced glial phagocytosis (Tenedini et al. 2025), which we believe is an early process in many neurodegenerative diseases. We investigate potentially common and diverging pathways required for axon and synapse maintenance to gain conceptual understanding of circuit maintenance, degeneration and its reversal.
References:
Tenedini FM, Yin C, Huang J, Dhiman N, Soba P*, Parrish JZ* (2025). Axon length-dependent synapse loss is mediated by neuronal cytokine-induced glial phagocytosis. PNAS, 122 (21) e2422752122. doi.org/10.1073/pnas.2422752122. (*co-corresponding authors)
Chen M, Xu L, Wu Y, Soba P*, Hu C* (2023) The organization and function of the Golgi apparatus in dendrite development and neurological disorders. Genes Dis 10:2425–2442. doi: 10.1016/j.gendis.2022.11.009 (*co-corresponding authors).
Hu C, Feng P, Chen M, Tang Y, Soba P (2022) Spatiotemporal changes in microtubule dynamics during dendritic morphogenesis. Fly (Austin) 16:13–23. doi: 10.1080/19336934.2021.1976033
Hu C, Kanellopoulos A, Richter M, Petersen M, Konietzny A, Tenedini F, Hoyer N, Cheng L, Poon C, Harvey K, Windhorst S, Parish JZ, Mikhaylova M, Bagni C, Calderon de Anda FC, Soba P (2020). Conserved Tao kinase activity regulates dendritic arborization, cytoskeletal dynamics and sensory function in Drosophila. J Neurosci 40 (9) 1819-1833; doi:10.1523/JNEUROSCI.1846-19.2020
Tenedini FM, Saéz Gonzáles M, Hu C, Pedersen L, Petruzzi MM, Wang D, Richter M, Petersen M, Szpotowicz E, Schweizer M, Sigrist S, Calderon de Anda F, Soba P (2019). Maintenance of cell type-specific connectivity and circuit function requires Tao kinase. Nat Comm 10(1), 3506. doi:10.1038/s41467-019-11408-1
Neuromodulatory control of circuit function and behavior
Circuits need to be functional to sustain behavior, and most larval behaviors are consistently maintained during development. This is particularly true for nociception, which is an evolutionary conserved mechanism to detect and avoid noxious stimuli. Similarly to vertebrates, Drosophila larval nociceptors are able to detect different modalities including nociceptive touch and heat. How behavioral responses to these distinct modalities are encoded at the network level has so far not been elucidated. We have recently uncovered a circuit and neuromodulatory mechanism encoding mechano-nociceptive escape behavior (Hu et al., Nat. Neurosci. 2017), which relies on the Drosophila Neuropeptide Y homolog sNPF. sNPF action on the identified circuit elements is specific to mechano-nocicieption, while thermo-nociception seems to be encoded by a different part of the network.
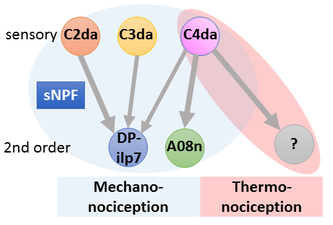
We are investigating how neuromodulation in this circuit regulates nociceptive responses using molecular, optogenetic and behavioral assays. Our studies aim to shed light on neuropeptide action in network function and behavioral action selection.
References:
Imambocus BN, Jiang Y, Schleyer M, Soba P (2025). Peptidergic top-down control of metabolic state-dependent behavioral decisions in a conflicting sensory context. bioRxiv, 2025.03. 12.642538. doi.org/10.1101/2025.03.12.642538.
Zhou F, Tichy A-M, Imambocus BN, Sakharwade S, Rodriguez Jimenez FJ, González Martínez M, Jahan I, Habib M, Wilhelmy N, Burre V, Lömker T, Sauter K, Helfrich-Förster C, Pielage J, Grunwald Kadow IC, Janovjak H, Soba P (2023) Optimized design and in vivo application of optogenetically functionalized Drosophila dopamine receptors. Nature Communications 2023 14:1 14:1–18. https://doi.org/10.1038/s41467-023-43970-0
Imambocus BN, Zhou F, Formozov A, Wittich A, Tenedini F, Hu C, Sauter K, Macarenhas Varela E, Herédia F, Casimiro AP, Macedo A, Schlegel P, Yang C-H, Miguel-Aliaga I, Wiegert JS, Pankratz MJ, Gontijo AM, Cardona A, Soba P (2022) A neuropeptidergic circuit gates selective escape behavior of Drosophila larvae. Curr Biol 32:149-163.e8. doi: 10.1016/j.cub.2021.10.069.
Hu C*, Petersen M*, Hoyer N*, Spitzweck B, Tenedini F, Wang D, Gruschka A, Burchardt LS, Szpotowicz E, Schweizer M, Guntur AR, Yang CH, Soba P (2017). Sensory integration and neuromodulatory feedback facilitate Drosophila mechanonociceptive behavior. Nat Neurosci 20(8):1085-95. (*equal contribution). doi: 10.1038/nn.4580